
#Gene therapy for scid trial#
If the immune system is not too bad, then the trial might not be a good fit.Īlso, most older children or young adults who have waning immunity or poor function after stem cell transplant may have more organ damage because of infection in their lungs or gastrointestinal tract. In addition, the immune system must be sufficiently defective so that the risk of going through the gene therapy is balanced out by how bad the immune system is. Because gene therapy is currently only offered in the context of a clinical trial, an older child or young adult who has already had a stem cell transplant must meet the criteria for that study, just like a newly diagnosed infant would. How can parents know if gene therapy is appropriate for an older child or young adult who has had one or two stem cells transplants already for SCID?Ī. A child might need a certain level of infection control or body organ function to participate.Īlso, because the outcomes of the matched sibling donor in hematopoietic stem cell transplant, or HSCT, (also known as bone marrow transplant) are so excellent, all of the current gene therapies are available only to those with no matched sibling donor. Therefore, the decision about whether gene therapy is appropriate or can be offered depends on each trial’s eligibility criteria for the child. Right now, all of the gene therapy that we listed is only available in clinical trials. Keeping in mind that gene therapy is only available for certain SCID types, what other ways can parents know if gene therapy is an appropriate treatment for their baby?Ī. Artemis SCID (caused by mutations in the DCLRE1C gene).ADA-SCID (caused by mutations in the ADA gene).X-linked SCID (caused by mutations in the IL2RG gene).Gene therapy is in clinical trials for genes responsible for the following SCID types: There are many genes that can cause SCID, but people haven’t developed genetic trials for all of those genes yet. Some of these stem cells grow into cells that form the immune system.Īlso, for any gene therapy to treat a specific genetic disease you have to know what the gene is.
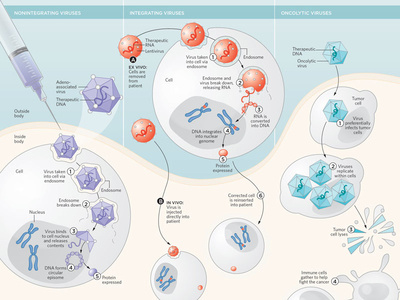
In the case of gene therapy for SCID, the cells that are being removed from the body are the hematopoietic stem cells, blood-forming cells that originate in the bone marrow. This is the type of gene therapy that is currently available in clinical trials for SCID. The other type involves removing cells from the body and injecting or delivering the genetic material into the cell using a vector and putting those genetically changed cells back into the body. One is the direct injection of the genetic material in the blood or organ or body cavity. There are two different ways that the genetic material can be delivered. Gene therapy is the term used for any treatment where you are delivering genetic material into the body. Please provide a brief explanation of gene therapy for SCID.Ī.
Pai is a pediatric hematologist/oncologist at Dana-Farber/Boston Children's Cancer and Blood Disorders Center where she evaluates patients with primary immunodeficiency for curative hematopoietic stem cell transplantation. Current projects in the laboratory include study of patients with immunodeficiency, particularly with severe combined immunodeficiency (SCID) and its variants and Wiskott-Aldrich syndrome, both pre and post-cellular therapy. Her laboratory focuses on the study of disorders of human T and B cell development and function, and treatment of these disorders by allogeneic hematopoietic cell transplantation and gene therapy. Sung-Yun Pai is co-director of the Gene Therapy Program, senior physician, and associate professor of pediatrics at the Harvard Medical School.


 0 kommentar(er)
0 kommentar(er)
